Formation and Evolution of Carbonate Phases upon Accelerated Carbonation of Mg-Oxides and Silicates
Oct 29, 2020
The PhD thesis defense of Kwon Bok Rodríguez will take pleace on Tuesday, november 3rd 2020, at 11:00 A.M. Thesis advisor: Dr. Ignacio Casanova Hormaechea Virtual Room: https://meet.google.com/ofw-akkg-zqi
This work discloses new insights into the formation and evolution of Mg-carbonates as well as carbonation processes of Mg-rich oxides and silicates with the aim of providing a safe and permanent anthropogenic CO2 storage, helping to tackle the worst effects of climate change. Carbonation reactions were carried in a purpose-built steam-mediated carbonation system at temperature and pressure ranges between 50-205ºC and 1 to 10 bar, respectively.
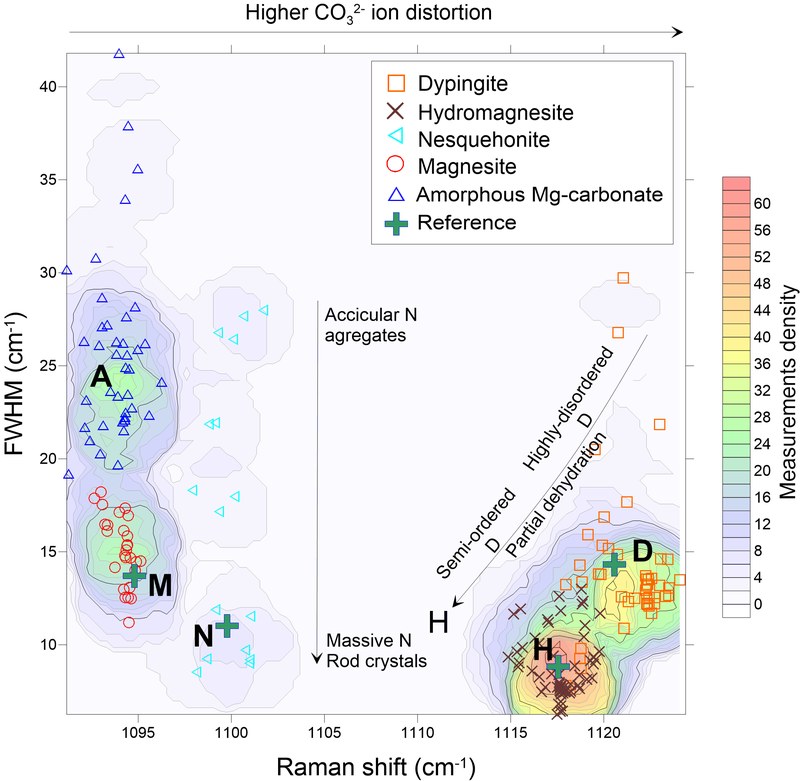
A hydrated amorphous Mg-carbonate was identified upon carbonation of Mg-rich silicates and oxides at 50ºC. Such material might have similar composition and thermal dehydration behavior as nesquehonite. Results from this thesis provided new insights into the enigmatic and yet unconstrained transition of such phase to less hydrated phases. The evolution of Mg-carbonate phases is mainly controlled by the slow dehydration kinetics of Mg2+ rather than evolving to a thermodynamically more stable or structurally similar phase. Despite it has been predicted that such material could straightforward transform into magnesite, it is strongly argued that such transition is greatly inhibited due to preferential nucleation pathways to less hydrated Mg-carbonate phases. Phases within the group Mg5(CO3)4·(OH)2·XH2O (11≤X≤4) allows a progressive dehydration whereas the MgCO3·nH2O (n≥0) seemingly not. It is proposed that the transition between hydrated amorphous Mg-carbonate to highly disordered dypingite-like phases could occur progressively as it dehydrates and crystallizes, forming dypingite-like phases.
The progressive evolution of dypingite-like phases is controlled by the removal of molecular water, inducing cell-shrinkage as well as ordering the internal structure heterogeneity, resulting in a crystalline hydrated structure with the name of hydromagnesite. This might explain the inconsistencies in the solubility and decomposition behavior data reported in the literature for such carbonate phases. No further dehydration is allowed within this group, entailing a significant kinetic barrier in order to allow the transition from hydromagnesite to magnesite.
Results from this work shed light into the yet enigmatic evolution of Mg-carbonate phases. The understating of such processes is of paramount importance to accelerate the transition and/or dehydration kinetics among such phases and possibly unlocking preferential nucleation pathways.
Brucite carbonation was observed to occur at feasible conversion rates even under simulated flue gas conditions, highlighting the potential of mineral carbonation processes for direct combined CO2 capture and storage/utilization.
Carbonation of Mg-rich silicates remained a challenging field under the studied conditions, even for activated serpentine, despite its partial high-reactivity attributed to the presence of a highly-reactive amorphous Mg-rich phase. It was also found the presence of a poorly-reactive Mg-rich amorphous phase (formed upon activation of lizardite) which remained seemingly unreacted upon carbonation. Such observation might provide new insights into the yet unanswered low carbonation efficiencies for direct-carbonation of activated serpentine.
Similar carbonation yields were observed for brucite-bearing serpentinized dunite when compared to activated lizardite. Strategically sourcing serpentinized rocks with higher brucite contents will potentially increase the carbonation potential of such materials. Coexisting lizardite and/or forsterite were also observed to be partially carbonated.
Carbonation of enstatite and forsterite were also individually studied under conditions relevant to localized early Martian conditions. Enstatite dissolution was observed by the formation of a Si-rich passivating layer, where Si and Mg are heterogeneously distributed. Such observation is consistent with morphological changes within this layer, strongly suggesting an intergrowth of nucleating and growing Mg-carbonates with Si-rich phases.
Share: